Hello, welcome to the official website of Liaoning Huagao New Materials Co., Ltd.

E-mail Box: sales@lnhgxc.com

Blog
Leave a Message/Feedback
If you have any questions, please leave your message, or contact us. Thank you for your support and trust, we will wholeheartedly provide you with high quality products and services!
GuestbookAn Introduction to Zirconium's Properties and Its Application Areas
Time:
2021-07-12
I. An Introduction to the Properties of Zirconium
Zirconium, with the element symbol Zr, is located in Group IV-B of the periodic table. It has an atomic number of 40 and is a silvery-white transition metal. Zirconium’s surface readily forms a protective oxide layer that gives it a glossy appearance, making it visually similar to steel. While it exhibits excellent corrosion resistance, it dissolves in hydrofluoric acid and aqua regia. At high temperatures, zirconium reacts vigorously with both non-metallic and many metallic elements, forming solid solution compounds. Zirconium is highly ductile and easy to work into shapes such as sheets and wires. When heated, it can absorb significant amounts of gases like oxygen, hydrogen, and nitrogen, making it an ideal material for hydrogen storage applications. Moreover, zirconium boasts superior corrosion resistance compared to titanium, rivaling even niobium and tantalum in this regard.

Zirconium exists primarily in nature as a mineral, ranking 20th in abundance within the Earth's crust—more plentiful than common metals like copper, lead, nickel, and zinc. Yet it’s often referred to as a "rare metal" because its extraction process is relatively complex and economically challenging to implement on a large scale. Moreover, out of the more than 40 zirconium-hafnium ore deposits discovered so far, only about 10 are considered commercially viable for mining. Of these, only two minerals—zircon and baddeleyite—are actually used extensively in industrial applications.
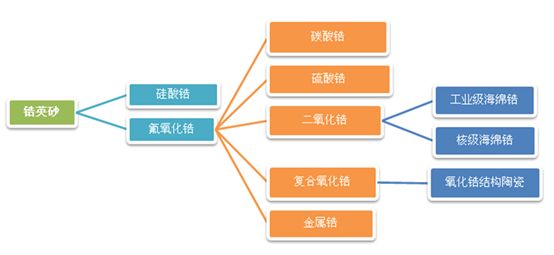
Zirconium products come in a wide variety of forms and types. Initially, zirconium exists as zircon sand, which, after undergoing treatments such as caustic soda processing and water washing, can be transformed into primary products like zirconium silicate and zirconyl chloride. By further processes—including calcination, chlorination, and reduction—these materials can be refined into advanced products such as zirconia and industrial-grade zirconium sponge. If the purification and separation technologies reach a high enough level, it becomes possible to produce nuclear-grade industrial-grade zirconium sponge. This material appears as a silvery-gray, sponge-like metal with a density of 6.49 g/cm³, a melting point of 1852 ± 2.001°C, and a boiling point of 4377°C. Zirconium is often referred to as "the first metal of the atomic age." Zirconium exhibits a steel-like appearance and boasts a brilliant metallic luster. Moreover, zirconium and its alloys demonstrate exceptional properties, including excellent dimensional stability, remarkable strength and toughness, outstanding oxygen absorption capabilities, superior resistance to erosion, robust performance under radiation exposure, and outstanding corrosion resistance.
II. Applications of Zirconium
As people’s understanding of zirconium continues to deepen, its applications are becoming increasingly widespread. While most people may still feel somewhat unfamiliar with zirconium, it has already woven itself into nearly every aspect of our daily lives—for instance, you’ll find it in the architecture around us, in essential ceramics used in households, in durable kitchen knives, and even in elegant decorative items. Moreover, zirconium plays a crucial role in cutting-edge technological fields such as nuclear reactors, defense industries, biomedical applications, aerospace, marine engineering, as well as oil and chemical processing, making it a material with immense potential for future innovation.

Compared to traditional metal elements like iron, copper, and nickel, zirconium has a lower density and a smaller thermal expansion coefficient. Additionally, zirconium boasts a significantly lower thermal neutron absorption cross-section—just 0.18 × 10⁻²⁴ cm². -28 ㎡) and excellent resistance to internal corrosion, making zirconium and its alloys highly promising for use in specialized fields such as the nuclear industry and aerospace. Currently, zirconium and its alloys are already well-established as cladding materials in nuclear reactors. Components fabricated from these materials include fuel cladding tubes, control rod guide tubes, pressure tubes, component boxes, and various structural materials. In China, large-scale nuclear power plants widely rely on zirconium-based materials—indeed, when generating nuclear power, a 100-megawatt facility consumes between 20 and 25 tons of metallic zirconium annually. Meanwhile, a single 30,000-horsepower nuclear submarine uses zirconium and zirconium alloys for both the cladding of nuclear fuel elements and pressure tubes, requiring an impressive 20 to 30 tons of zirconium.

From a military-industrial perspective, adding just one-thousandth of zirconium to steel can dramatically enhance its hardness and strength. Zirconium-containing materials—such as armor-grade steel, forged steel for artillery, stainless steel, and heat-resistant steel—are critical components in the production of armored vehicles, tanks, artillery pieces, and bulletproof plates, among other military equipment.
In the electrovacuum industry, zirconium powder is widely used to coat the surfaces of anodes and other heated components in electrovacuum devices and instruments. This coating effectively absorbs residual gases within vacuum tubes, enabling the creation of highly evacuated electron tubes and other electrovacuum instruments—thereby enhancing their quality and significantly extending their operational lifespan.
Zirconium powder is characterized by its low ignition point and rapid combustion rate, making it ideal as an initiating charge for detonators—advanced types of which can even explode underwater. When zirconium powder is combined with an oxidizer, it’s like adding fuel to fire: the resulting blaze produces a brilliantly dazzling light, making it an excellent material for crafting incendiary and illuminating projectiles.

Zirconium boasts exceptional corrosion resistance, effectively withstanding the corrosive attack of most organic acids, inorganic acids, strong alkalis, and even certain molten salts. As a result, zirconium materials are increasingly used in critical components of corrosive environments to significantly extend their service life. Today, zirconium is already widely adopted as a corrosion-resistant material in the chemical industry, with mature applications spanning areas such as heat exchangers, scrubbing towers, reactors, pumps, valves, and pipelines designed for corrosive media. For instance, zirconium alloy tubes—specifically those used for concentration and hydrolysis processes—have been successfully integrated into hydrogen peroxide production lines. Meanwhile, zirconium-based devices like pressure-reducing valves, agitators, and flow meters have also found practical use in industries such as fertilizer manufacturing, wastewater treatment, and dye production.
Biomedical materials are an emerging class of advanced high-tech materials developed in recent years, and bio-applicable alloys must exhibit excellent biocompatibility as well as superior corrosion resistance when exposed to biological fluid environments. Zirconium has gained significant attention from researchers due to its outstanding biocompatibility, elastic modulus similar to that of bone, and remarkable corrosion resistance.

Meanwhile, in fields such as space exploration, deep-sea exploration, and high-speed railways—for example, rocket launcher nozzles, lunar rover supports, thermal insulation panels used in aerospace equipment, as well as submarine engine impellers, blades, screws, and other components—zirconium and its alloys play a critical role. In China's zirconium industry, metallic zirconium will be the area with the greatest potential, the most promising highlight, and the brightest prospect for future development.
(Compiled from online sources)
Previous page
Previous page
